Matter is the substance that comprises everything in the universe. It is essentially everything that exists in your world, from planets to atoms and molecules.
At its most fundamental level, matter is made up of tiny particles you call atoms. These atoms further combine together to form molecules.
Atoms and molecules are two of the most basic units of matter; however, they are pretty different from each other.
An atom is the smallest unit of an element that can hold a particular chemical substance (e.g., carbon, nitrogen, oxygen). In contrast, molecules are a group of atoms bonded together by covalent bonds.
The key difference between atoms and molecules is that atoms are indivisible, while molecules are composed of smaller units called atoms. Apart from that, atoms are much smaller than molecules, and atomic masses are much greater than molecular masses.
Let’s discuss these two fundamental particles of matter in detail.
What Is An Atom?
Atoms are the smallest particles of an element that make up a molecule; they’re also the building blocks of matter.
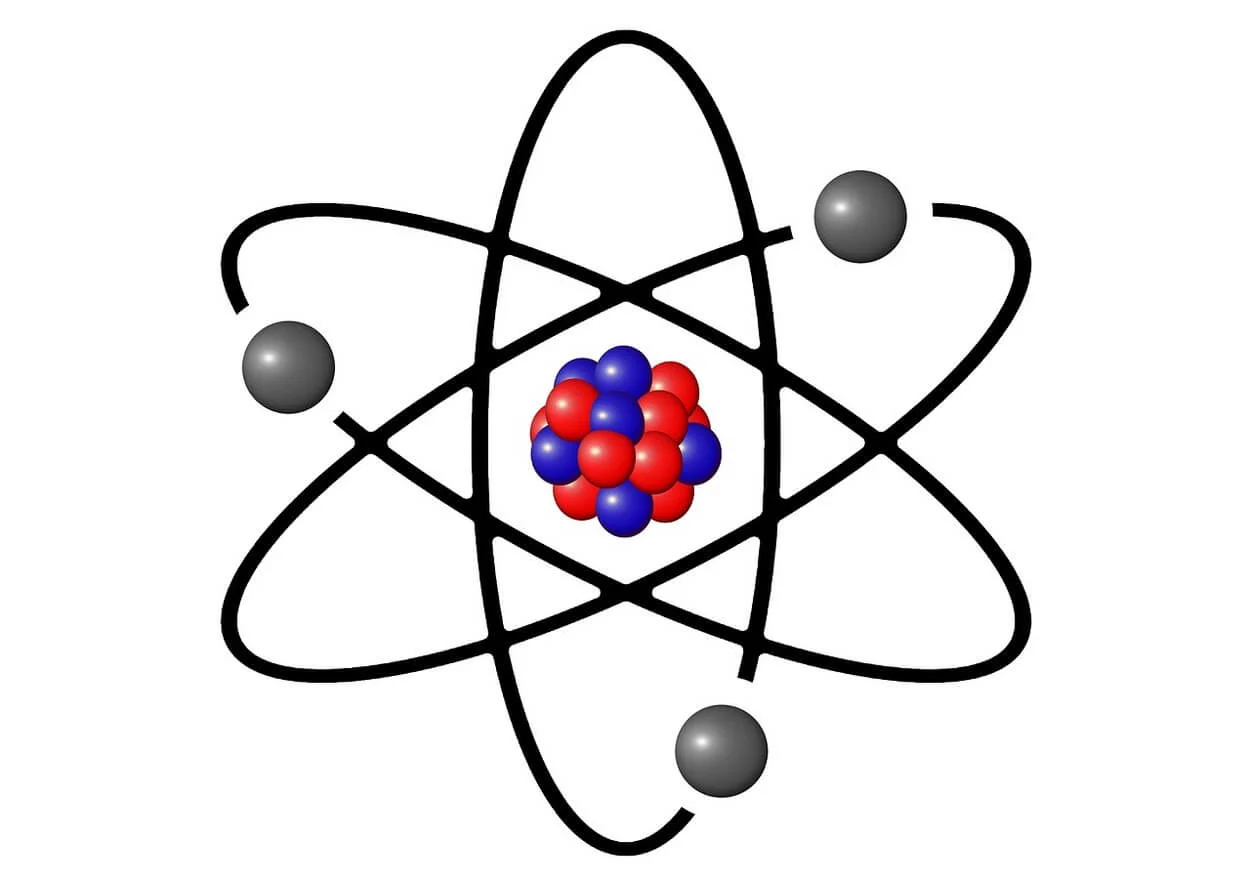
An atom consists of a nucleus (a small, dense nucleus) surrounded by electrons. Atomic weight is defined by how many protons are in the nucleus. Neutrons are a fairly insignificant part of an atom’s nucleus and don’t affect its properties.
The electrons orbiting the nucleus can be considered tiny planets orbiting the sun, except they’re way, way smaller! They have a negative electric charge and move around the nucleus in elliptical orbits.
An electron can only exist within certain boundaries called orbitals. The electron is said to be “free” or “unbounded within an outer orbital.”
But once it moves into an inner orbital, it becomes “bound” to the nuclei. Its energy levels fluctuate considerably due to quantum mechanics (the laws governing how matter behaves at very small scales).
Due to their electrically charged nature, atoms can attract other atoms or molecules with similar charges. This process is called ionization, creating new elements from simple atoms.
For example, when oxygen gas combines with nitrogen gas, we get nitrogen gas (plus some heat). This ionization process happens all the time in nature – think about moisture droplets coalescing with raindrops to create bigger drops or water vapor condensing into clouds.
What Is A Molecule?
Molecules, the smallest units of matter that can form substances, play an important role in everyday life. They are the building blocks of everything from insect wings to human blood cells.
Molecules come in all shapes and sizes, and various elements can be used to make them.
Molecules are composed of atoms. Atoms are the smallest particles of an element that have the property of holding together to form molecules.
The number of atoms in a molecule is called its atomic weight. There are almost 100 types of atoms in the human body, and over 100,000 different types of molecules.
Each molecule has specific chemical properties because the atoms are arranged in a specific way. Some molecules are very large, like proteins and DNA. Other molecules, like oxygen and nitrogen gas, are so small that you can’t see them with the naked eye.
The role of molecules is crucial to every aspect of our lives, from keeping us healthy to create the things we use daily.
How Many Atoms Combine Together To Form A Molecule?
A molecule is created when two or more atoms combine together to form a compound.
You need at least two atoms to make a molecule. Molecules can be small, like water molecules, or they can be large, like proteins.
Molecules play an important role in our everyday lives by absorbing sunlight to create energy, transporting drugs throughout the body, and clotting blood.
Difference Between An Atom And A Molecule
The following are some key differences between atoms and molecules:
- Atoms are the smallest units of an element that can exist in nature. They are made up of protons and neutrons in a nucleus, which orbits around the nucleus in shells.
- Molecules are composed of atoms, but the atoms are arranged into structures called molecules.
- Atoms can’t orbit around the nucleus as molecules do.
- Molecules are larger than atoms and can be formed from two or more atoms.
- Molecules are substances that can interact with each other and can form compounds or substances.
- Atoms cannot interact with each other and cannot form compounds or substances.
- Atoms are tiny and made up only of protons and neutrons, while molecules contain much more information about how they will function.
- Atoms tend to be more isolated from other atoms than molecules, giving them more specific properties.
- Atoms can be isolated from each other relatively easily, while Molecules are much more difficult to break down into their individual parts.
Here is the table of differences between atoms and molecules.
| Atoms | Molecules |
| An atom is the smallest component of an element and the most fundamental. | Molecules comprise two or more chemically bonded atoms. |
| It cannot exist freely in nature. | It can exist freely in nature. |
| Atoms are more reactive due to the presence of free electrons. | Molecules are stable and less reactive due to bonded electrons. |
| Protons, neutrons, and electrons make up an atom. | Molecules are made up of atoms. |
Watch this video clip further to clarify the difference between an atom and a molecule.
Examples Of Atom And Molecule
Atoms are the smallest particles of an element that make it up. They can be seen as the building blocks of matter.
There are a total of around 100 different atoms, but the most common ones on Earth are:
- Hydrogen (H)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- And phosphorous (P)
Molecules are the smallest matter that can be seen with the naked eye. Their chemical properties are unique, and they come in a variety of sizes and shapes.
Some common molecules you might encounter include:
- Water vapor (H2O)
- Carbon dioxide (CO2)
- Nitrogen gas (N2)
- And Oxygen gas (O2)
How Do You Know How Many Atoms Are In A Molecule?
In general, the number of atoms in a molecule is calculated by dividing the molecular mass by the total number of electrons in the molecule.
Many people are curious about how many atoms are in a molecule. To answer this question, scientists use mathematical formulas to calculate the number of atoms in a particular molecule.
There are many different types of molecules, so calculating the number of atoms for each can be quite complicated. However, chemists have developed some general rules to help them figure out how many atoms are in a particular molecule.
You can use a simple way to do this. All you need to do is divide the molecular weight (the mass of a molecule) by the atomic weight (the mass of an atom). This will give you the number of atoms in that particular molecule.
What Are The Different Types of Molecules?
There are three main types of molecules:
- Monoatomic (one atom)
- Diatomic (two atoms)
- Polyatomic (more than two atoms)
Each type of molecule has its distinctive properties and patterns of interactions with other molecules.
Monoatomic molecules are made up of one atom only. These molecules are very stable and difficult to break down, which is why they’re used in many chemical processes.
Monoatomic molecules are often used as starting points for creating more complex substances because they’re relatively non-reactive and easy to control.
Diatomic molecules consist of two atoms. They’re a bit more reactive than monatomic molecules and can generally be broken down into smaller pieces by various chemical reactions. Diatomic molecular substances typically react with other substances to form new compounds.
On the other hand, polyatomic molecules consist of more than two atoms. These larger molecules can’t be easily broken down into smaller chunks and require specialized chemicals to be turned into something useful.
Polyatomic molecules are often used in medical treatments because they have a wider range of abilities when interacting with different elements in the body.
What Are The Three Parts Of Atoms?
Atoms are the smallest particles that make up everything in the world. They are made of protons and neutrons, and these small particles make atoms powerful.
An atom has three parts: the nucleus, the electrons, and the space between them.
The nucleus is where all of the energy is stored for an atom. It is this core that contains protons and neutrons.
The electrons orbit around the nucleus. They aren’t really part of it, but they’re always close to it. This means that atoms can be different colors depending on how many electrons are present in a particular atom.
Are Humans Made Of Atoms?
Most scientists say we are, but there is still much debate on this topic.
Some people think that the molecules that make up our body are made of tiny particles called atoms. Others believe that we are actually composed of energy fields or quarks.
Final Thoughts
- Atoms and molecules are two very different things.
- Atoms are the smallest particles that make up materials like air, water, and rocks. They are made of protons and neutrons in a nucleus.
- Molecules are the large, organic compounds that we see all around us. Molecules usually have at least one atom and can contain hundreds or thousands of atoms.
- Atoms can be divided into tiny particles that are invisible to the naked eye, while molecules are larger and can be seen with the naked eye.
- Atoms have a specific weight determined by the number of protons in their nucleus. On the other hand, molecules can vary in weight based on the number of atoms they contain.