Sodium chloride, written as NaCl, is an ionic compound also called rock salt, common salt, table salt, or sea salt. It is found in the ocean and seawater. NaCl is created to combine two very compassionate elements that are 40 % sodium Na+ and 40% chloride Cl-.
Table salt, or NaCl(s), is a solid sodium compound, typically crystals. Each of the constituents of the complex lacks the energy necessary to move about in the crystalline structure. When a substance is listed as NaCl(aq), it is dissolved in water and split into positively and negatively charged ions that are encircled by water molecules.
It is commonly used in cooking, medicine, and the food industry for preserving, cleaning, toothpaste, shampoos, and deicing roadsides in the snowfall season; to keep patients from dehydrating, sodium chloride, an essential nutrient, is employed in healthcare.
How NaCl is Composed?
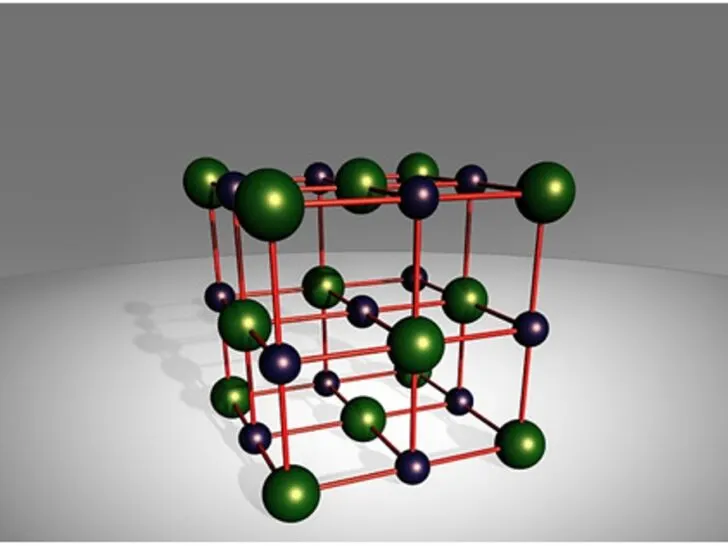
It is formed by the ionic bonding of one sodium cation (Na+) for every chloride ion (Cl-); hence the chemical formula is NaCl. When sodium atoms merge with chloride atoms, sodium chloride is formed. Table salt sometimes referred to as sodium chloride, is an ionic substance made up of 1:1 sodium and chloride ions.
Its chemical formula is NaCl. It is frequently employed as a food preservation and condiment. The weight of sodium chloride in grams per mole is denoted as 58.44g/mol.
The chemical reaction is:
2Na(s)+Cl2(g)=2NaCl(s)
Sodium (Na)
- Sodium is a metal having the symbol “Na” and its atomic number is 11.
- It has a relative atomic mass of 23.
- It is a delicate, silvery-whitish, and very reactive element.
- In the periodic table, it is in column 1(Alkali metal).
- It has a single electron in its outer shell, which it donates, creating a positively charged atom, a cation.
Chloride (Cl)
- Chloride is an element with the symbol “Cl” and 17 is its atomic number.
- Chloride ion has an atomic weight of 35.5g.
- Chloride is present in the halogen group.
- Carl Wilhelm Scheele discovered it.
Structure of Sodium Chloride
Who Discovered Sodium Chloride?
In 1807, a British chemist named Humphry Davy used electrolysis to separate NaCl from caustic soda.
It is a very soft, silvery-white metal. Sodium is the sixth-largest element on the planet, but it only makes up 2.6% of its crust. It is a highly reactive element that has never been found free.
Properties Of Sodium Chloride
Sodium chloride, commonly known as a salt, represents a 1:1 ratio of sodium and chloride ions. With atomic weights of 22.99 and 35.45 g/mol.
- It is easily dissolved in water, and its solubility is 36g per 100g.
- It is highly reactive with water.
- They are white crystalline solids with a bitter taste.
- NaCl is a good conductor of electricity.
- It reacts with acids to create hydrogen gas.
Some of the chemical properties of NaCl are tabulated below:
| Properties | Values |
| Boiling point | 1,465°c |
| Density | 2.16g/ cm |
| Melting point | 801°c |
| Molar mass | 58.44 g/mol |
| Classification | Salt |
| Atomic weight | 22.98976928 amu |
| Group in the periodic table | 1 |
| Group name | Alkali metal |
| Color | Silver white |
| Classification | Metallic |
| Oxidation state | 1 |
| Classification | 5.139eV |
What is NaCl Solid(s)?
It is a solid sodium chloride usually found in the form of crystals.
We usually know it as table salt. It is hard, transparent, and colorless.

What is NaCl Aqueous (aq)?
The aqueous form means that the compound has been dissolved in water and separated into positive ions (Na+) and negatively charged ions (cl-) surrounded by a water molecule.
Difference Between NaCl (s) and NaCl (aq)
| NaCl (s) | NaCl (aq) |
| It is solid sodium and is usually found in crystal form. The “s” symbolizes solid, which means hard. It is commonly known as table salt, and its typically used in food seasoning and preservatives. It is hard transparent and colorless. NaCl in the solid state does not conduct electricity. Sodium is a neutral compound with a Ph value of 7. It is an essential mineral for the body and brain. It is used in medicines, baby products, and anti-aging creams. | The “aq” symbolizes aqua, which means soluble in water. NaCl (aq) is an aqueous sodium chloride solution; in other words, it is a salt and liquid mixture. Pure sodium chloride mixture is colorless. It conducts electricity because it is a soluble ionic compound. It is used in medicine, such as saline drops. In the solution of salt and water, water act as the solvent, whereas NaCl is the solute. The solution where water is the solvent is called an aqueous solution. NaCl AQ solution is called Brine. |
Uses of Sodium Chloride NaCl
Sodium chloride (salt) is an essential part of our life. It is mainly used in cooking, the food industry, and the making of other household items, and it is also used in medicines and industrial areas.
NaCl has numerous uses, such as:
Sodium in Food
Salt is a widely used mineral in every food. It has a void of calories and nutrients. However, some table salt has iodine properties. Table salt contains 97% of sodium chloride.
- It is used as a food seasoning/flavor enhancer.
- Natural food preservative
- Preserving meat
- Creating brine for marinating food
- Salt is also used in fermenting for particular foods like a pickle.
- Sodium is a naturally occurring mineral found in several fruits and vegetables.
- It is also used as a meat tenderizer and enhances the flavor
Use of Sodium in the Food Industry
NaCl is beneficial in the food industry, as well as food production and processing. It is used as a preservative and also used as a color maintenance agent.
Sodium is used to control fermentation by stopping the growth of bacteria. It is also used in bread, bakery items, meat tenderizers, sauces, spice mixes, different types of cheese, fast food, and ready-made objects.
Health Benefits of Sodium Chloride
The body needs sodium, and salt is the primary source of NaCl and plays a prominent role in our health. It supports your body in absorbing calcium, chloride, sugar, water, nutrients, and amino acids. NaCl is good for the digestive system and is also a component of gastric juice.
It is essential for brain development; sodium deficiency directly affects brain performance, resulting in confusion, dizziness, and fatigue. It helps control blood pressure and volume and maintains an average fluid balance.
During the summer season, dehydration and muscle cramps are common. Sodium helps in the hydration and relaxation of muscles. Sodium helps to relieve indigestion and heartburn. NaCl helps to maintain the fluid level and electrolysis in the body.
Other Health Benefits
- Sodium is the essential ingredient of anti-aging creams for fighting the signs of aging.
- It is also in moisturizing lotions and crack creams and has healing properties.
- Sodium is widely used in soaps, shampoo, and baby care products to control dryness and itching.
- NaCl is also used in shower soaps and gel, and it can treat some skin conditions and help to remove dead skin.
- It plays a very influential role in oral hygiene; sodium helps remove stains from teeth and make them look white.

Medical Uses of Sodium Chloride
Sodium chloride is also used in medicines, such as injections and saline drops.
1. Intravenous injection (IV drips)
These drips are used to treat dehydration, and electrolyte imbalance, mixed with glucose or sugar. It helps to regulate the amount of fluid in the body.
2. Saline nasal spray
It is used to irrigate the nose, and the nasal sinus antrum gives moisture and lubricant to the nasal passage and treats nasal dryness and congestion.
3. Saline flush injection
It is a mixture of water and sodium (AQ) used to clean and remove any blockage by intravenous lines and deliver medicine directly into the vein.
4. Ear wash/irrigation
It is used to cleanse ear wax and blockage.
5. Eye drops
It can be used to treat eye redness, swelling, and discomfort, and keep your eyes moist.
6. Sodium chloride Inhalation (nebulizer)
NaCl is used in the nebulizer solution to help loosen mucus from the chest and improve breathing.
Household Uses of NaCl
It helps to remove stains and grease. It is usually used in dishwashing liquids, detergents, cleaners, soaps, and toothpaste. Sodium is used to clean roadside snow after a heavy snowstorm.
NaCl can make plastic, paper, rubber, glass, household bleach, and dyes. It is also used in fertilization. Sodium is also present in perfumes, deodorants, bleach, drain cleaners, nail polish, and remover.
Possible Side Effects of NaCl
Salt is essential for human bodies, but excessive consumption may not be suitable for health. It can lead to the following risks:
- High blood pressure
- Stroke
- Liver and kidney diseases.
- Heart failure
- Severe thirst
- Calcium slackens
- Fluid retention
Sodium is not suitable for hair; it may damage hair growth and the scalp. It also affects color and reduces hair moisture.
Conclusion
- Sodium chloride (NaCl) is a significant compound found in various forms.
- It exists as a solid (NaCl(s)) and as a solution when dissolved in water (NaCl(aq)).
- NaCl(s) is solid table salt, while NaCl(aq) forms a saline solution when dissolved in water.
- Sodium chloride contains sodium (Na) and chloride (Cl) ions in equal amounts.
- It is used in cooking, food preservation, medicine, and industry.
- Sodium is highly reactive, reacting with water and oxygen. It has properties like conductivity.
- It has health benefits, aiding in digestion, hydration, and muscle function.
- Sodium chloride is used in household products, medical treatments like IV drips, and industrial processes.
- Excessive consumption can lead to health risks like high blood pressure and kidney issues.
- Overall, sodium chloride is essential in daily life, from cooking to industry, but moderation is key to avoiding health problems.
FAQs
What is the difference between s and aq?
In our world, substances can exist in three main states: solid, liquid, or gas. When representing these states, we use “s” for solid, “l” for liquid, and “g” for gas. Additionally, “aq” stands for aqueous, which means a substance is dissolved in water.
What does the S stand for in chemistry?
In chemistry, “S” stands for sulfur, an element with the atomic number 16. Sulfur is located in period 3 and group 16 of the periodic table. When we use a lowercase “s,” it typically represents the solid state of an element.