The most widely utilized gas fuel worldwide is “natural gas”. It would be simple to comprehend the entire idea of natural gas and how its components or forms are used daily in businesses and homes for various purposes by brushing up on your hydrocarbon chemistry.
Understanding the special qualities and uses of liquefied natural gas (LNG), liquefied petroleum gas (LPG), and natural gas liquids (NGLs) is becoming more and more crucial as we negotiate the complexity of our modern energy needs.
Natural gas is converted into LNG, LPG, and NGLs, which are then used to fuel vehicles, power businesses, and heat homes.
Methane is not an NGL or an LPG; it is a dry natural gas. As LNG, it may stand alone. Ethane, propane, regular butane, isobutane, and natural petrol make up the NGLs to review. They are made either by the extraction of natural gas or the refining of crude oil.
However, despite having seemingly identical acronyms, they have different functions, go through different production processes, and are used in a range of industries.
Keep reading to learn more about LNG, LPG, and NGLs’ nature, function, and properties, along with the possible differences between the three.
What Is A Natural Gas?
Methane and ethane are the two main saturated light paraffins found in natural gas, which is a hydrocarbon combination.
It has been utilized as a fuel for automobiles, power, heating, and cooking. In and of itself, natural gas is a fossil fuel that is derived from petroleum.
Methane is the primary hydrocarbon in natural gas, though there are other hydrocarbons as well. It was created millions of years ago when sedimentary rock formations covered the remains of dead marine animals that had fallen to the ocean floor, and under pressure and heat, they were turned into gas.
There are different types of natural gases. Let’s look at the table below that provides detailed information on the types.
| Types of Natural Gas | Composition | Chemical Formula | Primary Function(s) |
|---|---|---|---|
| Natural Gas (NG) | Primarily, Methane (CH4) | CH4 | Used for heating, electricity generation, and as a clean fuel for various applications |
| Liquefied Natural Gas (LNG) | Mostly Methane, with some Ethane, Propane, and Butane | CH4, C2H6, C3H8, C4H10 | Transported and stored in a liquid form for more efficient long-distance transportation and storage |
| Liquid Petroleum Gas (LPG) | Mostly Propane (C3H8) and Butane (C4H10) | C3H8, C4H10 | Used as a fuel for heating, cooking, and transportation, especially in areas where natural gas pipelines are not available |
| Natural Gas Liquids (NGLs) | Comprising Ethane (C2H6), Propane (C3H8), Butane (C4H10), and Pentane (C5H12) | C2H6, C3H8, C4H10, and C5H12 | Used as feedstocks for petrochemical production, including the manufacturing of plastics and chemicals |
What Is LNG (Liquified Natural Gas)?

Liquified natural gas, or LNG, is natural gas that has been transformed into a liquid condition for simpler transportation and storage.
Natural gas that has been cooled down to a liquid state is cooled to -162ºC for safe transport and in an unpressurized condition is known as liquefied natural gas.
Methane (CH4), which makes up the majority of natural gas, is normally found in a gaseous condition at room temperature and atmospheric pressure. However, natural gas can be converted into a thick, energy-dense liquid that occupies a lot less area than its gaseous form by chilling it to extremely low temperatures.
Traits of LNG
The distinctive qualities of LNG, such as its ability to change into a thick liquid state and its benefits to the environment, make it an essential part of the world’s energy market and numerous sectors.
- Liquefaction Process: The conversion of natural gas into LNG is achieved through a process known as liquefaction. During this process, natural gas is cooled and condensed to a temperature where it changes from a gas to a liquid.
- Volume Reduction: LNG is highly energy-dense and takes up about 1/600th of the volume of natural gas in its gaseous state.
- Transportation: LNG is typically transported in specially designed cryogenic tanker ships or trucks equipped with double-walled, vacuum-insulated tanks.
- Storage: LNG is stored in insulated tanks at import and export terminals, industrial facilities, or aboard LNG carriers.
- Applications: LNG is used for a variety of applications, including electricity generation, heating, and as a clean fuel for various industries.
- Environmental Benefits: LNG is considered a cleaner-burning fuel compared to other fossil fuels like coal and oil. It produces fewer emissions of greenhouse gases and air pollutants when burned for energy generation or transportation.
What Is LPG (Liquid Petroleum Gas)?

The flammable hydrocarbon gases propane and butane, which are used as fuel for gas stoves, cooktops, and LPG cars, are what is commonly referred to as LPG, or liquefied petroleum gas.
It is a flexible and frequently used energy source that principally consists of the gases propane (C3H8) and butane (C4H10).
It comes from the processing of natural gas or the refining of crude oil. LPG is a significant fuel source for numerous applications due to its convenience, portability, and clean-burning qualities.
Some key characteristics of LPG are as follows:
- Odorless
- Highly flammable
- Heavier than air
What Is NGL (Natural Gas Liquids)?
The components of natural gas that are liquidized after being removed from the gaseous state are known as natural gas liquids (NGL).
To separate the liquids into their various components, the liquids must first be removed from the natural gas. A hydrocarbon is a natural gas liquid.
Ethane, propane, butane, isobutane, and natural gasoline are all included in the category of hydrocarbons known as natural gas liquids (NGLs). The number of carbon atoms in each NGL’s molecular chain serves as a defining characteristic between them.
Natural gas liquids can be divided into three categories based on their vapor pressures:
- Low (condensate)
- Intermediate (natural gasoline)
- High (liquefied petroleum gas)
Difference Between LNG (liquified natural gas), LPG (liquid petroleum gas), and NGLs (natural gas liquids)

Liquid natural gas (LNG), liquid petroleum gas (LPG), and natural gas liquids (NGLs) are all hydrocarbon-based compounds, but they vary in terms of their chemical makeup, intended use, and physical characteristics.
LPG is primarily a mixture of propane (C3H8) and butane (C4H10), LNG is primarily made up of methane (CH4), and NGLs are a group of hydrocarbon compounds that include ethane (C2H6), propane (C3H8), butane (C4H10), and pentane (C5H12).
While LPG is delivered in a liquid condition under moderate pressure and vaporizes into a gas when released, LNG is in a liquid state at extremely low temperatures (about -260°F or -162°C) and atmospheric pressure.
During processing, NGLs are frequently recovered from natural gas streams and separated from methane using fractionation.
Among its many uses, LPG is utilized for home heating, cooking, hot water systems, car fuel, and industrial processes. In contrast, LNG is employed for energy generation, home heating, and as a clean fuel for a variety of businesses and modes of transportation.
Ethane is utilized in the petrochemical sector, along with propane and butane, as well as other NGLs as ingredients in industrial processes and chemicals.
Other Types of Gases
Butane
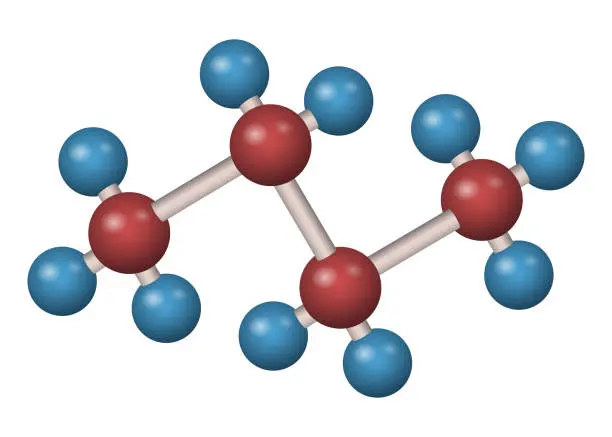
The molecular formula for the hydrocarbon gas butane is C4H10. It belongs to the alkane family of hydrocarbons and is distinguished by having ten hydrogen atoms and four carbon atoms.
N-butane (common butane) and isobutane (2-methylpropane) are the two isomeric forms of butane, and they have somewhat different molecular structures and characteristics.
Due to its high energy density, it is frequently used as a fuel for portable stoves, lighters, and torches.
Pentane
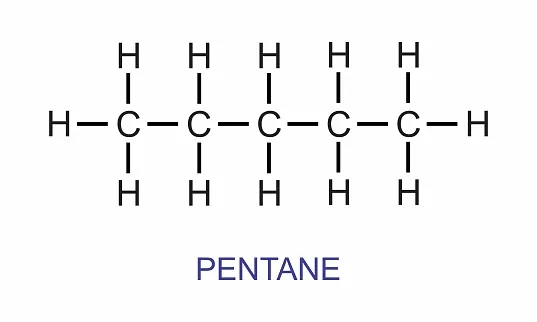
A hydrocarbon gas known as pentane, whose chemical formula is C5H12. Its five carbon atoms and twelve hydrogen atoms define it as a member of the alkane family of hydrocarbons.
Pentane, like other alkanes, is commonly found at ambient temperature and atmospheric pressure in a gaseous condition. Because the chain of carbon atoms in this substance is continuous, it is referred to as a straight-chain alkane.
In the lab and some industrial operations, it serves as a solvent. Additionally, it can be used as a feedstock to make other compounds.
Conclusion
- A natural gas liquid is an NGL. They are parts of the natural gas stream that separate into a liquid mixture when cooled. This mixture separates into fuels like propane, ethane, and butane which you may be familiar with with pressure and heat.
- Many common goods, including plastics, textiles, cellphone components, heating fuels, and even newborn diapers, are made with them.
- LNG is natural gas that has been liquefied by cooling it to minus 260 degrees Fahrenheit. The main component of LNG is methane. This is done to make storage and travel easier and safer. After arriving at its destination, LNG is heated in import facilities to be transformed back into gas for pipeline transmission.
- Propane, regular butane, and isobutane are three of the pure natural gas liquids that we mentioned earlier that also happen to be offered as liquefied petroleum gas.
- The word “petroleum” is added since these goods can also be produced through the refining of crude oil. The remaining natural gas comes from the mixture we just discussed.

